Dr. Zelenko’s Latest Study on Hydroxychloroquine – Approved
Researchers at the Henry Ford Health System find the early use of hydroxychloroquine against coronavirus cuts death rates in half; reaction from Fox News medical contributor Dr. Marc Siegel.
****************************************************************************************************
One of the South Carolina General Assembly’s staunchest advocates for individual liberty is advancing hydroxychloroquine as an effective treatment for the coronavirus pandemic after interviewing a doctor who experienced success treating patients with the drug.
State representative Stewart Jones of Laurens, S.C. recently interviewed Michelle L. Hagenbuch, a family medicine specialist based in Clinton, S.C.
FROM The CDC
CS237187-CMedicines for the Prevention of Malaria While Traveling Hydroxychloroquine (Plaquenil™)
PDFSpecifications on Hydroxychloroquine
CDC COVID-19 Last Updated: July 17, 2020
Chloroquine or Hydroxychloroquine
- The Panel recommends against the use of chloroquine or hydroxychloroquine for the treatment of COVID-19, except in a clinical trial (AII).
- The Panel recommends against the use of high-dose chloroquine (600 mg twice daily for 10 days) for the treatment of COVID-19 (AI).The combination of hydroxychloroquine plus azithromycin (AIII), because of the potential for toxicities.
- Lopinavir/ritonavir (AI) or other HIV protease inhibitors (AIII), because of unfavorable pharmacodynamics and because clinical trials have not demonstrated a clinical benefit in patients with COVID-19.
**************************************************************************************************************
Successful Treatment Strategy of Turkey against Covid-19 Outbreak read more
******************************************************************************************************
Hydroxochloroquine – How Does it Work
Lectures by Dr. Mobeen Sayed and Dr. Roger Sehault
*******************************************************************************************************
The War Against Hydroxychloroquine

The Association of American Physicians & Surgeons filed a motion for a preliminary injunction to compel release to the public of hydroxychloroquine by the Food & Drug Administration (FDA) and the Department of Health & Human Services (HHS), in AAPS v. HHS, No. 1:20-cv-00493-RJJ-SJB (W.D. Mich.). Nearly 100 million doses of hydroxychloroquine (HCQ) were donated to these agencies, and yet they have not released virtually any of it to the public.
Millions of Americans fear attending political gatherings, religious services, and even large family get-togethers without the availability of early treatment if they were to contract COVID-19. Why should Americans have to wait until they or a loved one is on a ventilator before they gain access to medication to overcome this virus?
“Why does the government continue to withhold more than 60 million doses of HCQ from the public?” asks Jane Orient, M.D., the Executive Director of AAPS. “This potentially life-saving medication is wasting away in government warehouses while Americans are dying from COVID-19.”
War on Science and Medicine

The current front in the war against science and medicine, and hence against doctors and patients, is the war to suppress available, affordable, safe preventives and treatments for COVID-19, as patients continue to die and economic carnage continues.
Ironically, “anti-science” is the accusation hurled at any who question the narrative: We cannot live normally but must mask, isolate, and practice “social distancing” until we have effective treatment, proved by accepted peer-reviewed randomized controlled trials (RCTs), or the just-around-the-corner vaccine.
Science requires honest data, honest scientists, replication of results, ethical methods, and open discussion.
The American Medical Association and many other professional organizations demand “peer-reviewed science” before they will recommend or even accept the re-purposing of a long-established anti-malaria drug to treat or prevent COVID-19. In Arizona, use of hydroxychloroquine (HCQ) for prophylaxis in medical workers is “strictly forbidden” by Executive Order of Gov. Doug Ducey unless peer-reviewed evidence becomes available. In all but four states, prescribing or dispensing HCQ for COVID-19 is restricted.
We have known about COVID-19 for about four months; it can take years to plan, complete, and publish a randomized, double-blind controlled study. However, in 2005, the CDC Special Pathogens Branch described three mechanisms by which chloroquine might work and have both a prophylactic and therapeutic role in coronavirus infections. More than 20 relevant studies have been published in journals indexed in PubMed between Jan 28 and April 20, 2020.
AAPS concludes that: “the safety of Hydroxychloroquine is well documented. When the safe use of this drug is projected against its apparent effect of decreasing the progression of early cases to ventilator use, it is difficult to understand the reluctance of the authorities in charge of U.S. pandemic management to recommend its use in early COVID-19 cases.”
Additionally, AAPS is compiling observational results reported from China, France, South Korea, Algeria, and the U.S. Of 2,333 patients treated with HCQ, 2,137 or 91.6 percent improved clinically. There were 63 deaths, all but 11 in a single retrospective report from the Veterans Administration where the patients were severely ill.
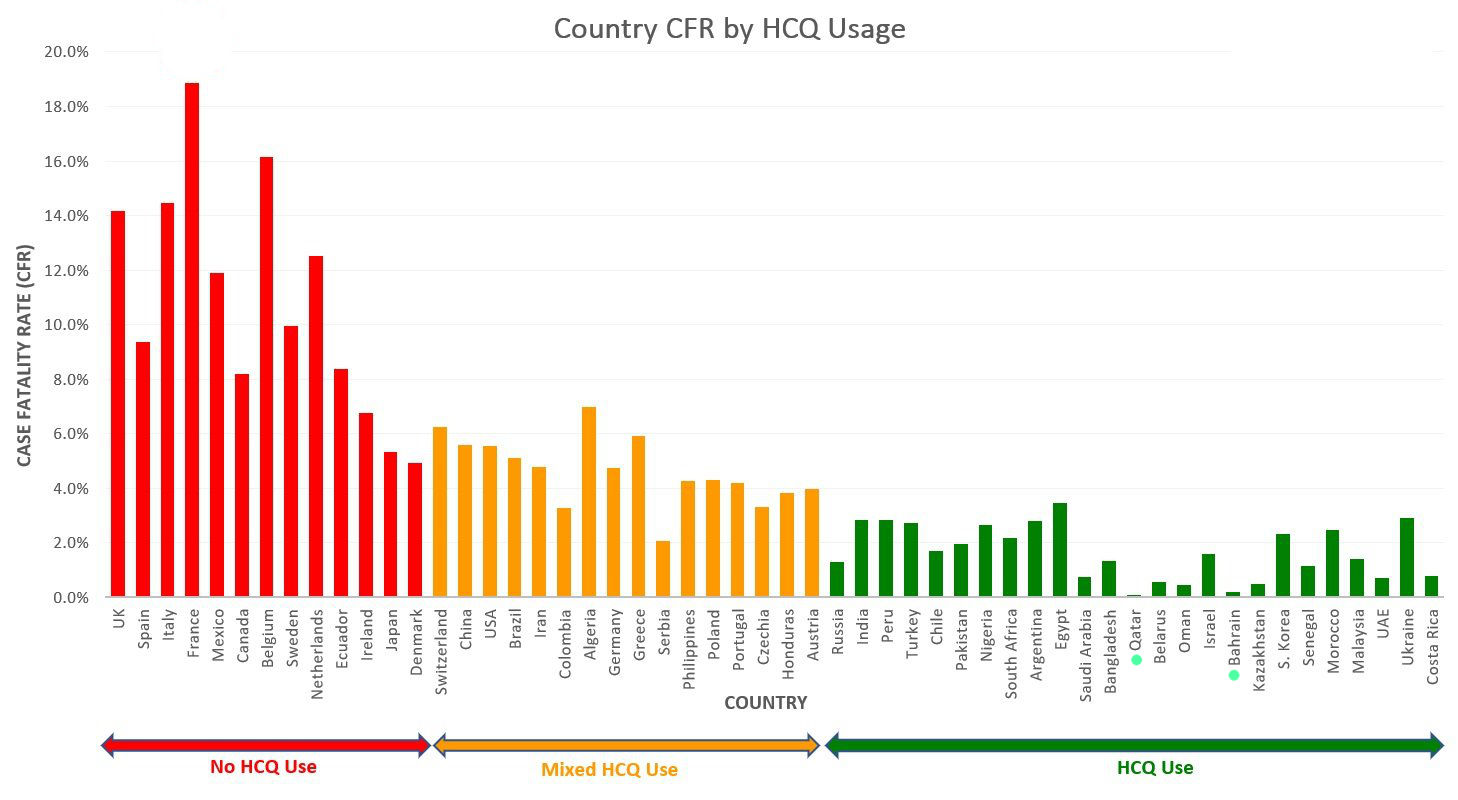
Apr 27 data show that U.S. COVID-19 death rates are at least eight times higher than in countries with early and prophylactic use of HCQ. READ MORE
**************************************************************************************************************
Country Fatality Rate by HCQ useage
********************************************************************************************8
Qatar


