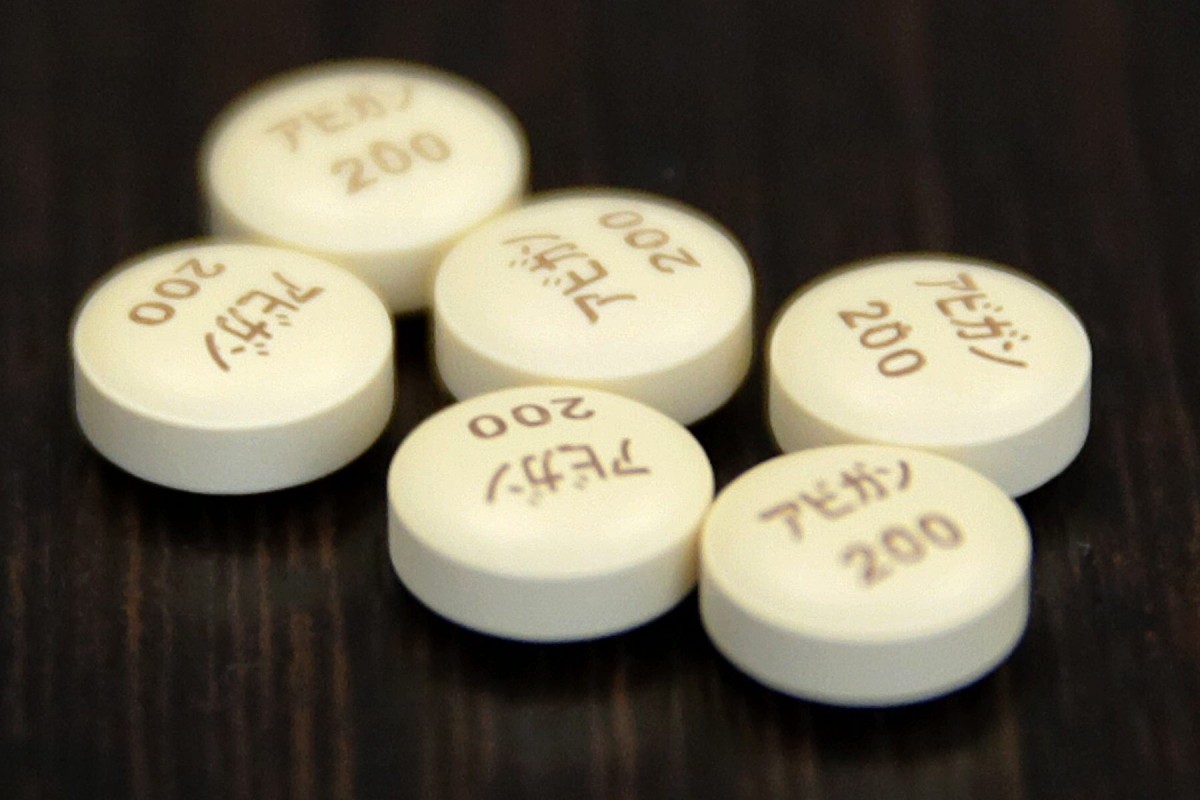
Trials of Japanese anti-flu drug Avigan to begin on Philippine coronavirus patients
- News follows shipment of 199,000 tablets of the drug from Japan
- Expert says the testing process could take three to six months
on Monday.
The Philippine government said on Friday it was fast-tracking the trial, which involves a test group of 100 people.
The announcement came after a shipment of 199,000 tablets of Avigan, sent by Japan as part of a grant-in-aid, arrived in Manila on Thursday.
While the Japanese embassy has stressed the effectiveness of the drug – which was intended to treat an outbreak of novel or re-emerging influenza virus infections not treatable by other antiviral drugs – has not been established against the coronavirus, some experts are optimistic it could play a role in treating patients with Covid-19.
The Japanese government approved the manufacture of Avigan, also known as favipiravir, in 2014, but it never went to market.
Health reform advocate Dr. Anthony Leachon said it was hoped that Avigan could be used to kill the virus in infected patients – contrasting it with a vaccine, which produces immunity in non-infected people.
If the trials are successful, the drug could be cheaply manufactured locally under licence by its Japanese maker, Fujifilm Toyama Chemical, Leachon said. The Philippines is one of at least 43 countries taking part in the trial.


Since the patent of Avigan’s active ingredient, favipiravir, expired last year, a generic version could even be produced by local drug companies, added Leachon, who worked as medical director for Pfizer Philippines for 16 years.
However, he cautioned there was a testing process of three to six months ahead. Even if the drug passed the tests and was put onto the market, he said there would then be a “post marketing surveillance trial” for side effects. This was “when a lot of drugs are pulled out”, he said, citing the arthritis painkiller Vioxx, which was withdrawn due to serious cardiovascular side effects.

Reviews of the drug have been mixed. A month ago, Kyodo News cited a clinical trial by Fujita Health University involving 89 patients who were divided into two groups – the first taking it as soon as their infections were confirmed and the second taking it six days later.
Professor Yohei Doi, who led the study, said the virus disappeared on the sixth day from 66.7 per cent of those who took Avigan immediately. However, among those who started taking it on the sixth day, 56.1 per cent immediately showed a similar recovery on the initial dose.


On average, fever subsided after 2.1 days and 3.2 days for the first and second groups, he noted.
However, Doi said the trial size was too small to yield statistically meaningful results.
Taiwan’s Centre for Disease Control has noted that in clinical studies conducted in Japan using monkeys, Avigan was found to have caused “early embryonic deaths” and was therefore not advisable for pregnant women. It could raise blood uric acid levels. Men were advised not to have intercourse for seven days after treatment.
In India, Mumbai-based drug firm Glenmark Pharmaceuticals is already marketing its version of favipiravir under the brand name FabiFlu. Because the original clinical trials conducted in Japan called for a patient to ingest 18 pills (of 200 mgs each) on the first day, Glenmark released in the market a 400 mg pill “to reduce the pill burden of Covid-19 treatment”.
News of the clinical trial came as Russian envoy to Manila Igor Khovaev on Friday said his country was “ready to supply vaccines to the Philippines” or invest with a local partner to produce them locally.
Foreign Secretary Tedoro Locsin Jnr responded on Twitter that he had “profound gratitude for Russia’s offer and your warm friendship”.
In May, Philippine health secretary Francisco Duque said China’s Sinopharm had “offered to include us in the global clinical trial of their newly-developed vaccine”.
Additional reporting by Reuters
Trials of Japanese anti-flu drug Avigan to begin on Philippine coronavirus patients
- News follows shipment of 199,000 tablets of the drug from Japan
- Expert says the testing process could take three to six months
on Monday.
The Philippine government said on Friday it was fast-tracking the trial, which involves a test group of 100 people.
The announcement came after a shipment of 199,000 tablets of Avigan, sent by Japan as part of a grant-in-aid, arrived in Manila on Thursday.
While the Japanese embassy has stressed the effectiveness of the drug – which was intended to treat an outbreak of novel or re-emerging influenza virus infections not treatable by other antiviral drugs – has not been established against the coronavirus, some experts are optimistic it could play a role in treating patients with Covid-19.
The Japanese government approved the manufacture of Avigan, also known as favipiravir, in 2014, but it never went to market.
Health reform advocate Dr. Anthony Leachon said it was hoped that Avigan could be used to kill the virus in infected patients – contrasting it with a vaccine, which produces immunity in non-infected people.
If the trials are successful, the drug could be cheaply manufactured locally under licence by its Japanese maker, Fujifilm Toyama Chemical, Leachon said. The Philippines is one of at least 43 countries taking part in the trial.


Since the patent of Avigan’s active ingredient, favipiravir, expired last year, a generic version could even be produced by local drug companies, added Leachon, who worked as medical director for Pfizer Philippines for 16 years.
However, he cautioned there was a testing process of three to six months ahead. Even if the drug passed the tests and was put onto the market, he said there would then be a “post marketing surveillance trial” for side effects. This was “when a lot of drugs are pulled out”, he said, citing the arthritis painkiller Vioxx, which was withdrawn due to serious cardiovascular side effects.

Reviews of the drug have been mixed. A month ago, Kyodo News cited a clinical trial by Fujita Health University involving 89 patients who were divided into two groups – the first taking it as soon as their infections were confirmed and the second taking it six days later.
Professor Yohei Doi, who led the study, said the virus disappeared on the sixth day from 66.7 per cent of those who took Avigan immediately. However, among those who started taking it on the sixth day, 56.1 per cent immediately showed a similar recovery on the initial dose.
On average, fever subsided after 2.1 days and 3.2 days for the first and second groups, he noted.
However, Doi said the trial size was too small to yield statistically meaningful results.
Taiwan’s Centre for Disease Control has noted that in clinical studies conducted in Japan using monkeys, Avigan was found to have caused “early embryonic deaths” and was therefore not advisable for pregnant women. It could raise blood uric acid levels. Men were advised not to have intercourse for seven days after treatment.
01:44
Links between brain disorders and coronavirus infections under investigation in the UK
In India, Mumbai-based drug firm Glenmark Pharmaceuticals is already marketing its version of favipiravir under the brand name FabiFlu. Because the original clinical trials conducted in Japan called for a patient to ingest 18 pills (of 200 mgs each) on the first day, Glenmark released in the market a 400 mg pill “to reduce the pill burden of Covid-19 treatment”.
News of the clinical trial came as Russian envoy to Manila Igor Khovaev on Friday said his country was “ready to supply vaccines to the Philippines” or invest with a local partner to produce them locally.
Foreign Secretary Tedoro Locsin Jnr responded on Twitter that he had “profound gratitude for Russia’s offer and your warm friendship”.
In May, Philippine health secretary Francisco Duque said China’s Sinopharm had “offered to include us in the global clinical trial of their newly-developed vaccine”.
Additional reporting by Reuters
Trials of Japanese anti-flu drug Avigan to begin on Philippine coronavirus patients
- News follows shipment of 199,000 tablets of the drug from Japan
- Expert says the testing process could take three to six months
on Monday.
The Philippine government said on Friday it was fast-tracking the trial, which involves a test group of 100 people.
The announcement came after a shipment of 199,000 tablets of Avigan, sent by Japan as part of a grant-in-aid, arrived in Manila on Thursday.
While the Japanese embassy has stressed the effectiveness of the drug – which was intended to treat an outbreak of novel or re-emerging influenza virus infections not treatable by other antiviral drugs – has not been established against the coronavirus, some experts are optimistic it could play a role in treating patients with Covid-19.
The Japanese government approved the manufacture of Avigan, also known as favipiravir, in 2014, but it never went to market.
Health reform advocate Dr. Anthony Leachon said it was hoped that Avigan could be used to kill the virus in infected patients – contrasting it with a vaccine, which produces immunity in non-infected people.
If the trials are successful, the drug could be cheaply manufactured locally under licence by its Japanese maker, Fujifilm Toyama Chemical, Leachon said. The Philippines is one of at least 43 countries taking part in the trial.


Since the patent of Avigan’s active ingredient, favipiravir, expired last year, a generic version could even be produced by local drug companies, added Leachon, who worked as medical director for Pfizer Philippines for 16 years.
However, he cautioned there was a testing process of three to six months ahead. Even if the drug passed the tests and was put onto the market, he said there would then be a “post marketing surveillance trial” for side effects. This was “when a lot of drugs are pulled out”, he said, citing the arthritis painkiller Vioxx, which was withdrawn due to serious cardiovascular side effects.

Reviews of the drug have been mixed. A month ago, Kyodo News cited a clinical trial by Fujita Health University involving 89 patients who were divided into two groups – the first taking it as soon as their infections were confirmed and the second taking it six days later.
Professor Yohei Doi, who led the study, said the virus disappeared on the sixth day from 66.7 per cent of those who took Avigan immediately. However, among those who started taking it on the sixth day, 56.1 per cent immediately showed a similar recovery on the initial dose.
On average, fever subsided after 2.1 days and 3.2 days for the first and second groups, he noted.
However, Doi said the trial size was too small to yield statistically meaningful results.
Taiwan’s Centre for Disease Control has noted that in clinical studies conducted in Japan using monkeys, Avigan was found to have caused “early embryonic deaths” and was therefore not advisable for pregnant women. It could raise blood uric acid levels. Men were advised not to have intercourse for seven days after treatment.
01:44
Links between brain disorders and coronavirus infections under investigation in the UK
In India, Mumbai-based drug firm Glenmark Pharmaceuticals is already marketing its version of favipiravir under the brand name FabiFlu. Because the original clinical trials conducted in Japan called for a patient to ingest 18 pills (of 200 mgs each) on the first day, Glenmark released in the market a 400 mg pill “to reduce the pill burden of Covid-19 treatment”.
News of the clinical trial came as Russian envoy to Manila Igor Khovaev on Friday said his country was “ready to supply vaccines to the Philippines” or invest with a local partner to produce them locally.
Foreign Secretary Tedoro Locsin Jnr responded on Twitter that he had “profound gratitude for Russia’s offer and your warm friendship”.
In May, Philippine health secretary Francisco Duque said China’s Sinopharm had “offered to include us in the global clinical trial of their newly-developed vaccine”.
Additional reporting by Reuters
Trials of Japanese anti-flu drug Avigan to begin on Philippine coronavirus patients
- News follows shipment of 199,000 tablets of the drug from Japan
- Expert says the testing process could take three to six months
on Monday.
The Philippine government said on Friday it was fast-tracking the trial, which involves a test group of 100 people.
The announcement came after a shipment of 199,000 tablets of Avigan, sent by Japan as part of a grant-in-aid, arrived in Manila on Thursday.
While the Japanese embassy has stressed the effectiveness of the drug – which was intended to treat an outbreak of novel or re-emerging influenza virus infections not treatable by other antiviral drugs – has not been established against the coronavirus, some experts are optimistic it could play a role in treating patients with Covid-19.
The Japanese government approved the manufacture of Avigan, also known as favipiravir, in 2014, but it never went to market.
Health reform advocate Dr. Anthony Leachon said it was hoped that Avigan could be used to kill the virus in infected patients – contrasting it with a vaccine, which produces immunity in non-infected people.
If the trials are successful, the drug could be cheaply manufactured locally under licence by its Japanese maker, Fujifilm Toyama Chemical, Leachon said. The Philippines is one of at least 43 countries taking part in the trial.


Since the patent of Avigan’s active ingredient, favipiravir, expired last year, a generic version could even be produced by local drug companies, added Leachon, who worked as medical director for Pfizer Philippines for 16 years.
However, he cautioned there was a testing process of three to six months ahead. Even if the drug passed the tests and was put onto the market, he said there would then be a “post marketing surveillance trial” for side effects. This was “when a lot of drugs are pulled out”, he said, citing the arthritis painkiller Vioxx, which was withdrawn due to serious cardiovascular side effects.

Reviews of the drug have been mixed. A month ago, Kyodo News cited a clinical trial by Fujita Health University involving 89 patients who were divided into two groups – the first taking it as soon as their infections were confirmed and the second taking it six days later.
Professor Yohei Doi, who led the study, said the virus disappeared on the sixth day from 66.7 per cent of those who took Avigan immediately. However, among those who started taking it on the sixth day, 56.1 per cent immediately showed a similar recovery on the initial dose.
On average, fever subsided after 2.1 days and 3.2 days for the first and second groups, he noted.
However, Doi said the trial size was too small to yield statistically meaningful results.
Taiwan’s Centre for Disease Control has noted that in clinical studies conducted in Japan using monkeys, Avigan was found to have caused “early embryonic deaths” and was therefore not advisable for pregnant women. It could raise blood uric acid levels. Men were advised not to have intercourse for seven days after treatment.
01:44
Links between brain disorders and coronavirus infections under investigation in the UK
In India, Mumbai-based drug firm Glenmark Pharmaceuticals is already marketing its version of favipiravir under the brand name FabiFlu. Because the original clinical trials conducted in Japan called for a patient to ingest 18 pills (of 200 mgs each) on the first day, Glenmark released in the market a 400 mg pill “to reduce the pill burden of Covid-19 treatment”.
News of the clinical trial came as Russian envoy to Manila Igor Khovaev on Friday said his country was “ready to supply vaccines to the Philippines” or invest with a local partner to produce them locally.
Foreign Secretary Tedoro Locsin Jnr responded on Twitter that he had “profound gratitude for Russia’s offer and your warm friendship”.
In May, Philippine health secretary Francisco Duque said China’s Sinopharm had “offered to include us in the global clinical trial of their newly-developed vaccine”.
Additional reporting by Reuters