So far, six patients have been treated, all of whom left the hospital virus free within 48 to 72 hours.
“From the first moment [that COVID-19 struck Israel] we realized plasma was an important tool for treating sick patients,” Rotstein told The Jerusalem Post. “The Health Ministry was reluctant even to see us collect the plasma. But at the end, the fruits are very delicious.”
Hadassah is currently running a clinical trial, testing the first-ever commercially produced plasma-derived immunoglobulin (IgG) serum for COVID-19, which Rotstein’s hospital created with Kamada Ltd., a local biopharmaceutical company. The trial is being run under the auspices of the Health Ministry.
So far, six patients have been treated, Rotstein said. All of them have left the hospital virus free within 48 to 72 hours. Another 12 patients were recently enrolled in the program.
“Instead of sending them to be hooked up to a ventilator, we sent them home,” Rotstein said.
He noted, however, that the hospital has learned from the trial the importance of administering the plasma the moment the patient shows signs of developing an acute case of the disease, such as pneumonia, strong coughing or high fever. Otherwise, he noted, that while the plasma may eradicate the virus, the patient may continue to suffer from its effects.
Earlier this summer, before the formal trial started, the serum was used to treat a critically ill 19-year-old patient who was being treated at Hadassah Hospital Ein Kerem. Although the patient stabilized for several days after receiving the serum, Rotstein said she was already too sick to be saved due to multiple previous underlying health conditions.
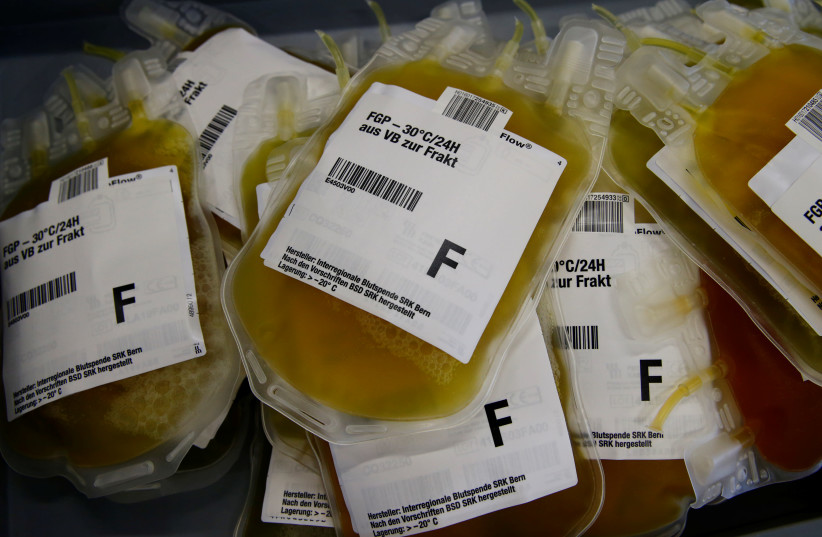
The antibody concentrate used at Hadassah was developed using plasma that the hospital harvested from recovered corona patients: those who had the disease and now test negative for the virus.
Those who develop any virus, including the novel coronavirus, develop special antivirus proteins or antibodies in their plasma, which could therefore help sick patients cope with the disease.
Plasma is the element of the blood that carries water, enzymes and blood cells throughout the body. It also carries the antibodies humans form to fight off disease and boost immunity.
The plasma works as a passive vaccine, which is when you are given antibodies formed by another patient who got the disease and developed them. This is in contrast to an active vaccine, when you are injected with a dead or weakened version of a virus that tricks your immune system into thinking that you have had the disease, and your immune system creates antibodies to protect you.
So far, Hadassah was able to collect 126 liters of plasma by working together with Jerusalem’s Beit Din Tzedek (Jewish court), which encouraged members of the haredi (ultra-Orthodox) community to donate their plasma after recovery. During the first and current coronavirus peaks, Jerusalem’s haredi neighborhoods have had some of the highest numbers of sick people.
Rotstein said that the vaccine, which could also be called a medicine, will likely continue to be targeted toward COVID-19 patients whose situation is worsening and need a booster to fight the disease. However, it may also be used prophylactically in cases where a high-risk patient contracts coronavirus and the hospital wants to stop the disease’s progression.
Kamada is running a parallel study of the serum and announced earlier this month that it had just recruited the first patients into its Phase I/II trial.
In America, the plasma treatment received “emergency use authorization” from the FDA.
The organization, explaining its decision, cited early evidence suggesting blood plasma can decrease mortality and improve the health of patients when administered in the first three days of their hospitalization.
The agency also said it determined this was a safe approach in an analysis of 20,000 patients who received the treatment, So far, 70,000 patients have been treated using blood plasma, the FDA said.
“It appears that the product is safe and we’re comfortable with that and we continue to see no concerning safety signals,” Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, told reporters in a conference call.
The World Health Organization on Monday was cautious about endorsing the use of recovered COVID-19 patients’ plasma to treat people who are ill, saying evidence it works remains “low quality” even as the United States issued emergency authorization for such therapies.
“There are a number of clinical trials going on around the world looking at convalescent plasma compared to the standard of care,” Soumya Swaminathan, WHO chief scientist, said.
“Only a few of them have actually reported interim results…and at the moment, it’s still very low-quality evidence,” she told a news conference.
Although Hadassah was the first to administer an antibody concentrate to a COVID-19 patient, several Israeli patients have been treated with frozen plasma via transfusion since the start of the first wave, also to much success.
MDA has been collecting plasma for more than 30 years. Plasma with antibodies was used to treat patients with SARS during the outbreak in 2002. In addition, Israel offered similar treatment to patients with West Nile fever.
Reuters contributed to this report.


